At a Glance
| Principal Activity: | Manufacturing, marketing and distribution of medical devices and consumables as well as the provision of after-sales service for all products. |
| Location (s): |
|
| Factory Building Built-Up Area: | 48,487.40 sq ft |
| Revenue (FYE 2022): | RM50,738,000 |
| Certification(s): |
|
| Number of Employees: | 100-200 |
| Core competency: |
|
| Website: | www.umedic.com.my |
| Contact Person: | Ng Sze Hui Email: shng@umedic.com.my |
Introduction
UMediC Group Berhad is an investment holding company. Through its subsidiaries, UMediC Healthcare Sdn Bhd (formerly known as UWC Healthcare Sdn Bhd) and UWHM Sdn Bhd (formerly known as UWC Healthcare MFG (M) Sdn Bhd), the company is principally involved in manufacturing, marketing and distribution of various branded medical devices and consumables as well as the provision of after-sales service for all products offered. With over 20 years of industry experience, UMediC Group currently exports to more than 30 countries.
Founded in 2002 through the incorporation of UMediC Healthcare, the company’s operation was primarily in the marketing and distribution of medical supplies and dental-related products and devices. Over the years, UMediC Group has diversified its marketing and distribution business to include various types of medical devices and consumables in light of its growing potential and demand for such products. Notably, the company is an authorised distributor for global well known healthcare brands such as Philips, GE healthcare, Mindray, Merit, Löwenstein etc.
In 2011, UMediC Group has ventured into the research and development (R&D) and manufacturing of medical consumables, carrying its own brand. In ensuring constant improvements on the R&D activities, the company has also established an in-house microbiology and chemical laboratory.
Products & Services
The main products manufactured by UMediC Group are HydroX prefilled humidifiers and AirdroX inhaler spacers.
| Product | Description |
|
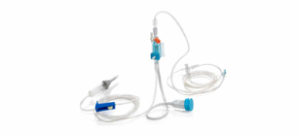
Prefilled humidifier
|
|
Inhaler spacer  |
|
Additionally, the medical devices supplied by UMediC Group include but not limited to the following:
| Product | Description |
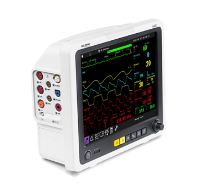
| Medical Devices (i) Patient monitor / MRI-compatible patient monitor |
|
| (ii) Defibrillator / AED
|
|
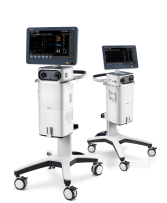
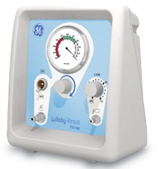
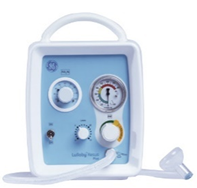
| (iii) Ventilator
|
|
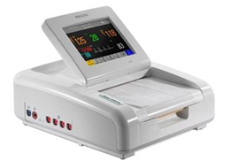
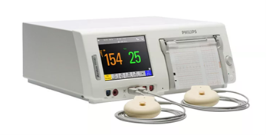
| (iv) Maternal and infant care Cardiotocography monitor |
|
| Infant resuscitation system
|
|
Infant radiant warmer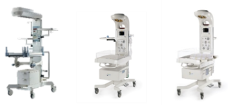  |
|
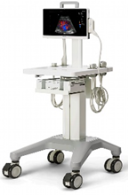
| (v) Ultrasound machine
|
|
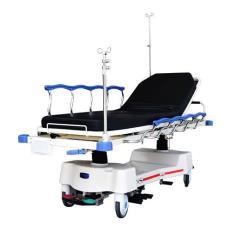
| (vi) Furniture Medical bed |
|
| Medical cart
|
|
| Medical Consumables (i)Pressure transducer kit |
|
| (ii) Arterial cannulation
|
|
| (iii) Arterial blood sampling systems
|
|
Achievements & Awards
In recognition of UMediC Group’s outstanding contribution to the sales of Philips’ medical products, the company was awarded the Best Country Order Growth, Patient Care and Monitoring Solutions and the Best Channel Partner Performance, Value Segment for Patient Monitors by Philips. On top of that, the company also received an appreciation award – Rising Star Award from Mindray, a Chinese multinational medical instrumentation manufacturer.
UMediC Group’s AirdroX inhaler spacers have registered with the Belgian Federal Agency for Medicines and Health Products. The product also won them the Gold Award under Best Innovation Award category, Malaysia Technology Expo 2020 Special Edition – COVID-19 International Innovation Awards.
Future Plan
UMediC’s future plans and business strategies are as follows:
- Construction of a new factory building
UMediC Groups’s current factory building has a built-up area of approximately 48,487.40 sq ft. The company is currently expanding its production capacity by building a new plant behind our current manufacturing facilities with a built up of 21,345.66 sq ft.
- Strengthening domestic presence and reach
UMediC Group plans to further strengthen its domestic presence through the setting up of one new marketing and distribution office each in the central area of Kuala Lumpur and Johor.
- Expanding product portfolio through the development of current and new brand distribution business
The demand for medical devices is expected to continue growing, and the company strives to offer a wider and more comprehensive selection of medical devices to end-customers. The company intends to distribute more medical devices in different specifications, price ranges and features, in order to fulfil the different requirements of our end-customers including public and private hospitals, other healthcare service providers as well as non-medical business entities.
- Development and commercialisation of new manufacturing products
Holding on to the aim to expand the manufacturing segment and also to develop and commercialise more products of own brand, UMediC Group has identified new products such as sterile water for inhalation, prefilled nebulisers, digital oxygen flowmeters and humidifier humidity sensors, which are targeted to commercialise in the next two years. These new products will be added to the existing list of own brand product offerings and cross-marketed to the company’s existing clientele.
Written in Feb 2023
Disclaimer:
Every effort is made to provide accurate and complete information in this article. However, InvestPenang makes no claims, promises or guarantees about the accuracy, completeness, or adequacy of the contents and expressly disclaims liability for errors and omissions of this article.